The electrolytic reduction-coupled super-stable mineralization process was used to recover 91.1 % of Cu 2+ in the treatment of actual electroplating wastewater, meeting the prescribed threshold for polluted water.. The process leads to positive economic outcomes, with significant reductions in medicine dosage and sludge volume, while also generating profits from recovered metals.. The automobile industry consumed 9 million metric tons of lead in 2012 for lead-acid batteries. Recycling lead from spent lead-acid batteries is not only related to the sustainable development of.
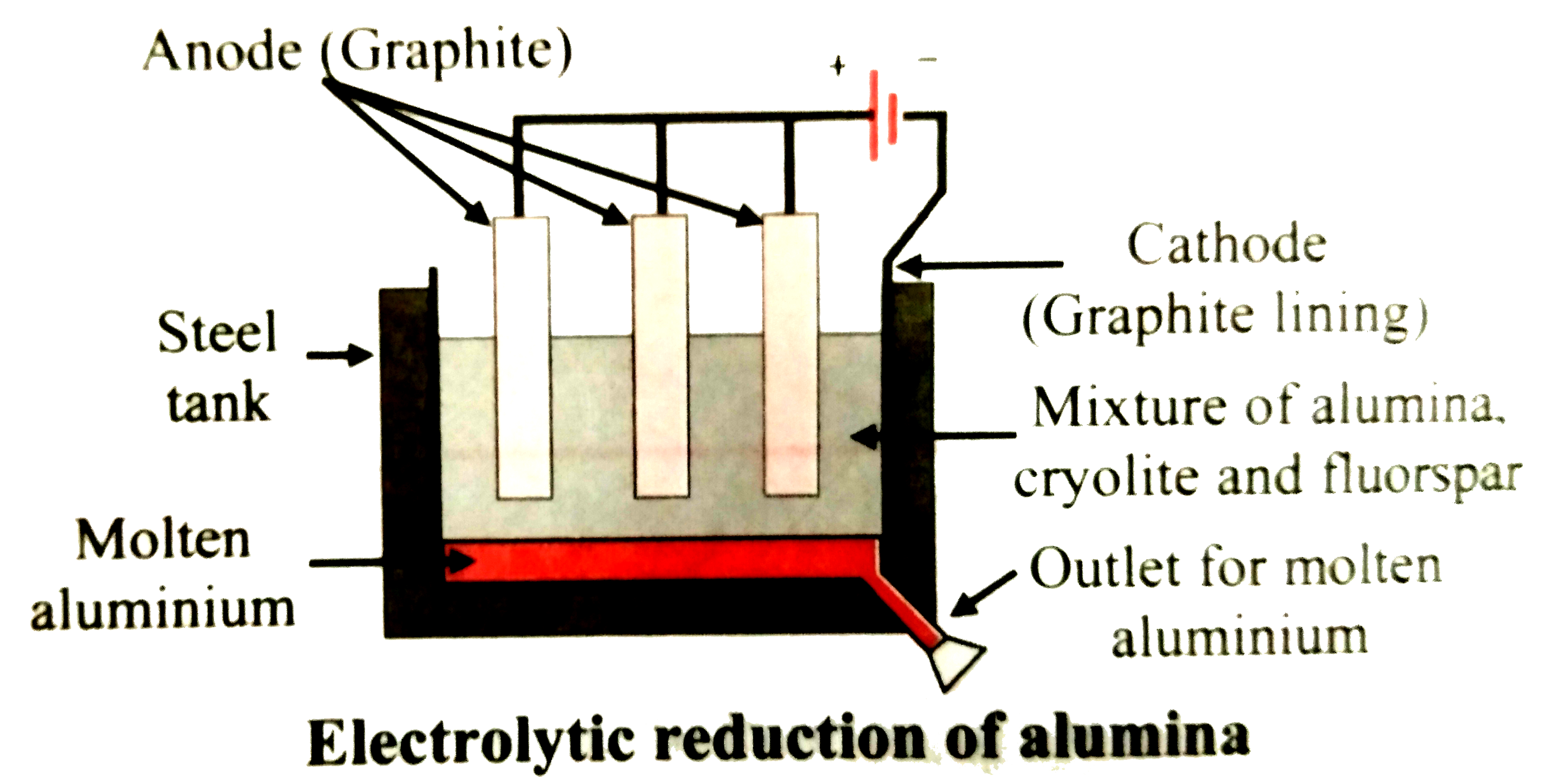
The adjacent figure represents electrolytic reduction of alumina.

Mechanistic studies of the electrochemical CO2toformate conversion on... Download Scientific

Electrochemical CO2 Reduction to Hydrocarbons on a Heterogeneous Molecular Cu Catalyst in
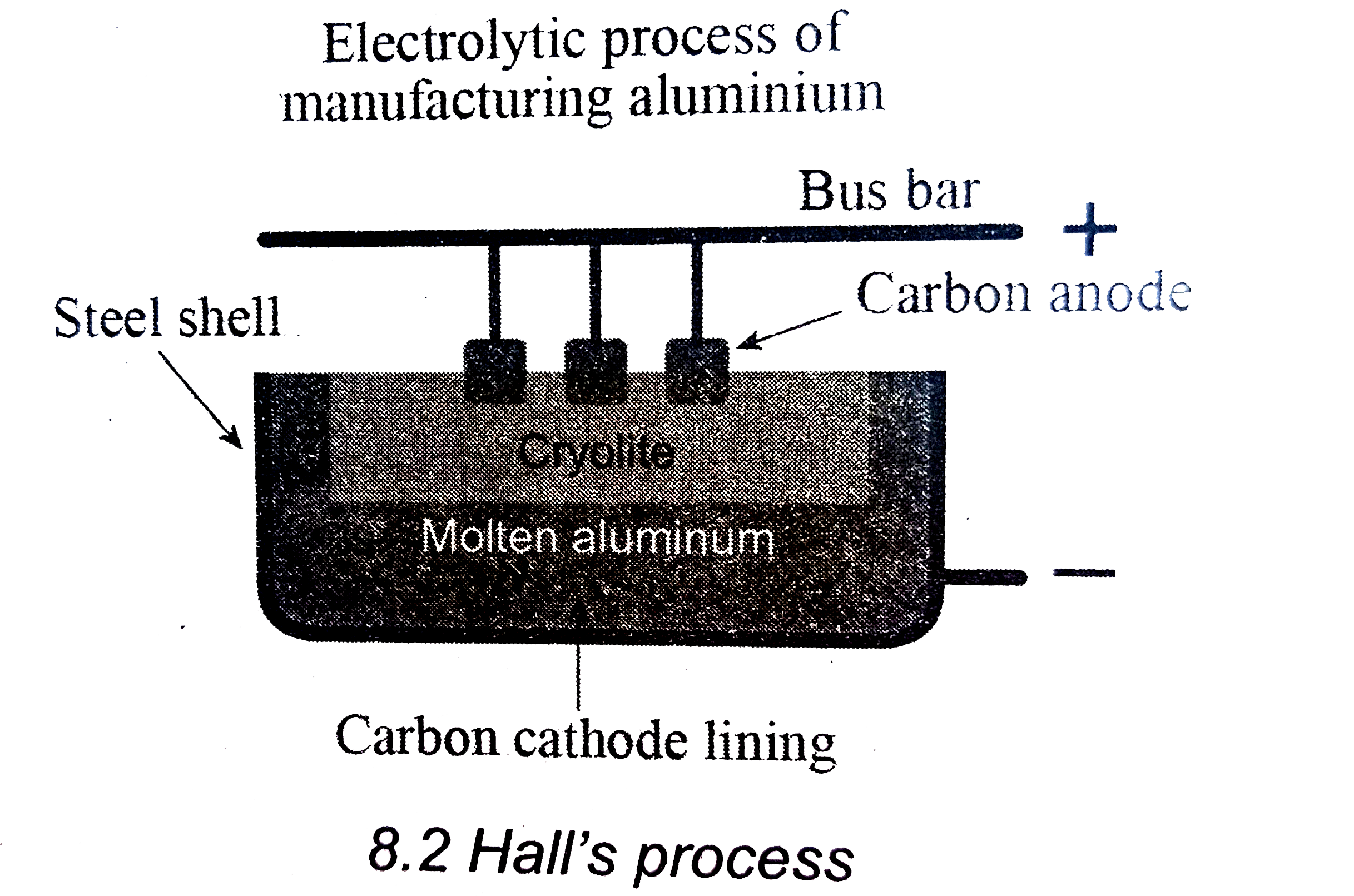
Explain the Hall's Process of electrolytic reduction of alumina with d

The electrolytic reduction of nitrobenzene in strongly acidic medium produces

Electrolytic reduction system. Download Scientific Diagram
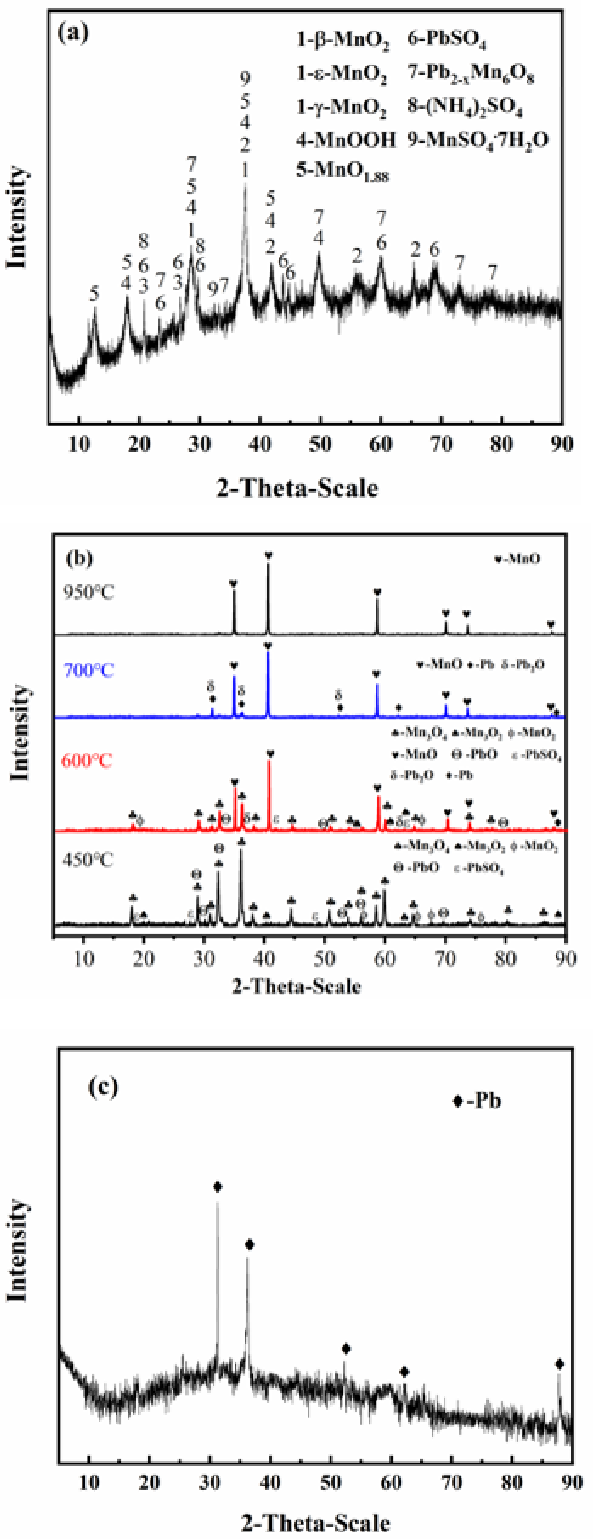
Figure 2 from Enhanced Removal of Pb from Electrolytic Manganese Anode Slime by Vacuum
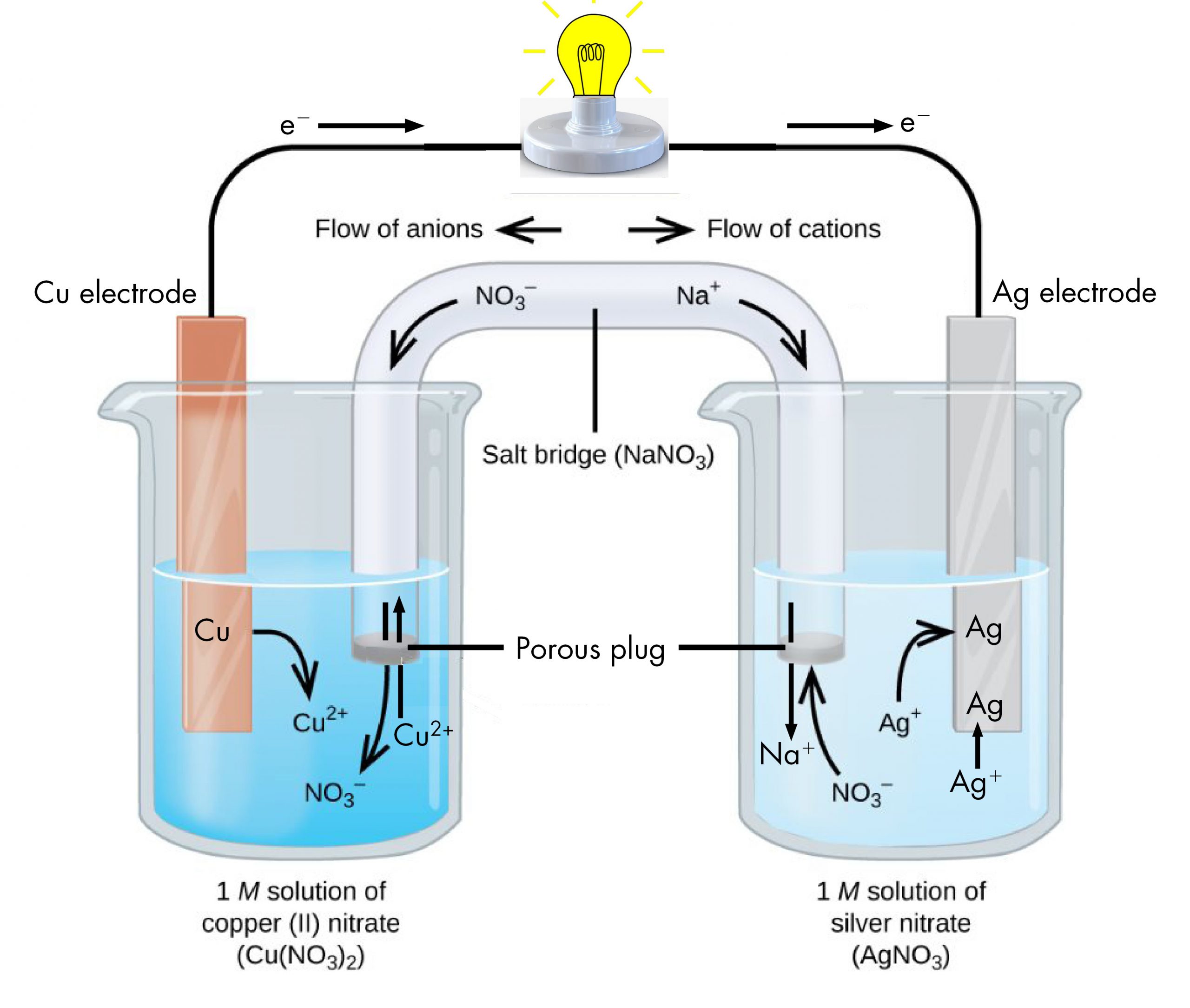
Copper Electrolytic Cell

Conventional Nd electrowinning comprises direct electrolytic reduction... Download Scientific

Electrolyte Effects on CO2 Electrochemical Reduction to CO Accounts of Chemical Research
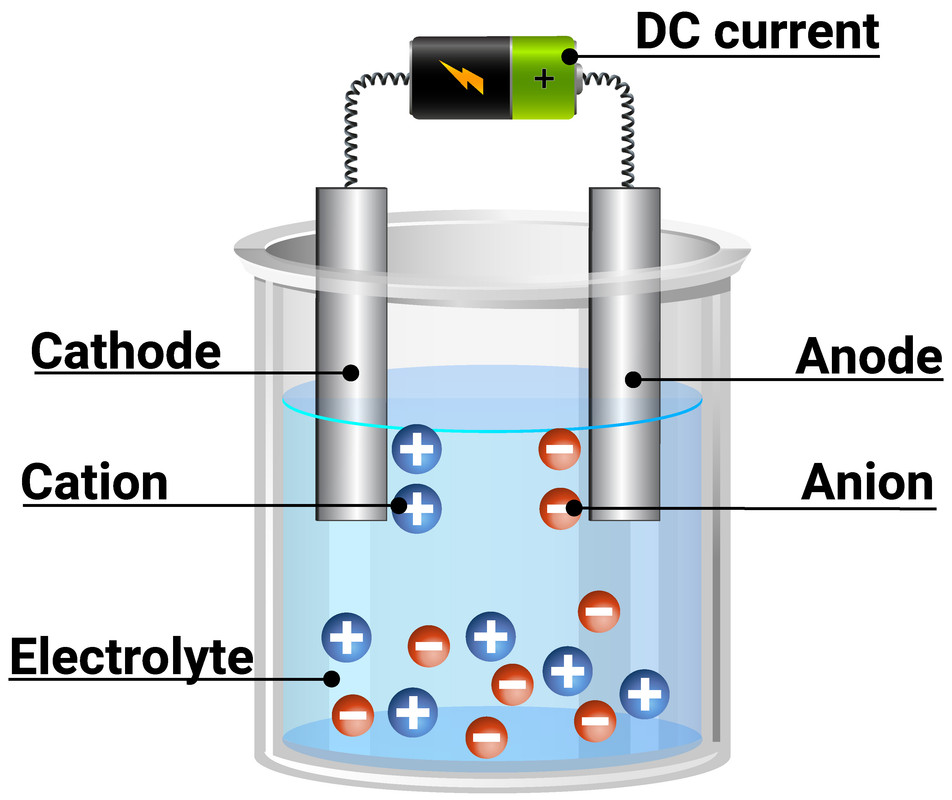
Circuit Diagram Electrolysis

Difference Between Electrolytic Reduction and Refining Compare the Difference Between Similar

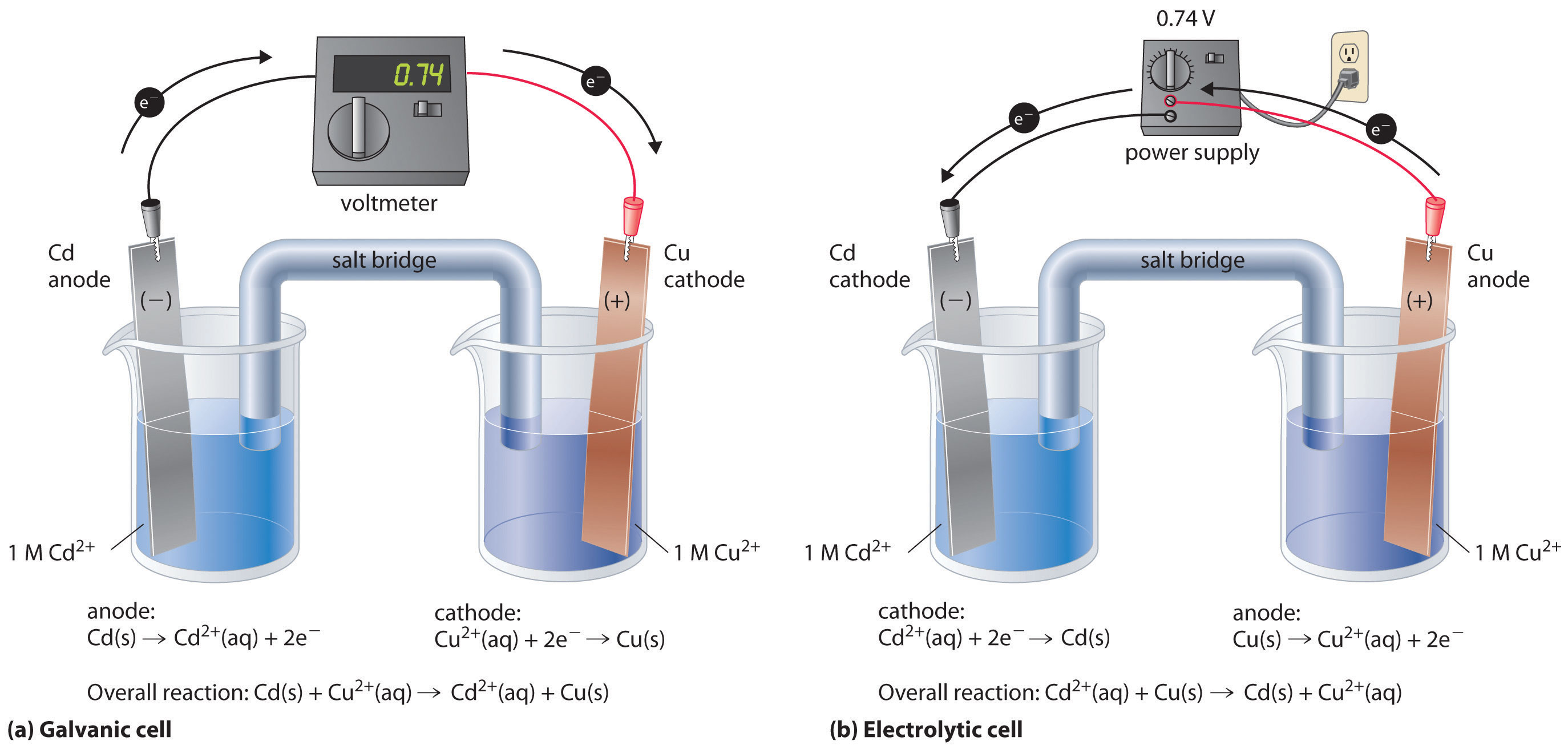
Where does reduction happen in electrolysis?
Chapter 19.7 Electrolysis Chemistry LibreTexts

Electrolysis of molten NaCl Oxidation half and reduction half cell reactions in electrolytic
59 Write the reactions at cathode and anode during electrolytic reduction of aluminium oxide.

The standard reduction potential of Pb and Zn electrodes are 0.126 and 0.763 volts

5.6 Day 41 Electrolysis; Commercial Batteries Chemistry LibreTexts

Electrolytic reduction system. Download Scientific Diagram
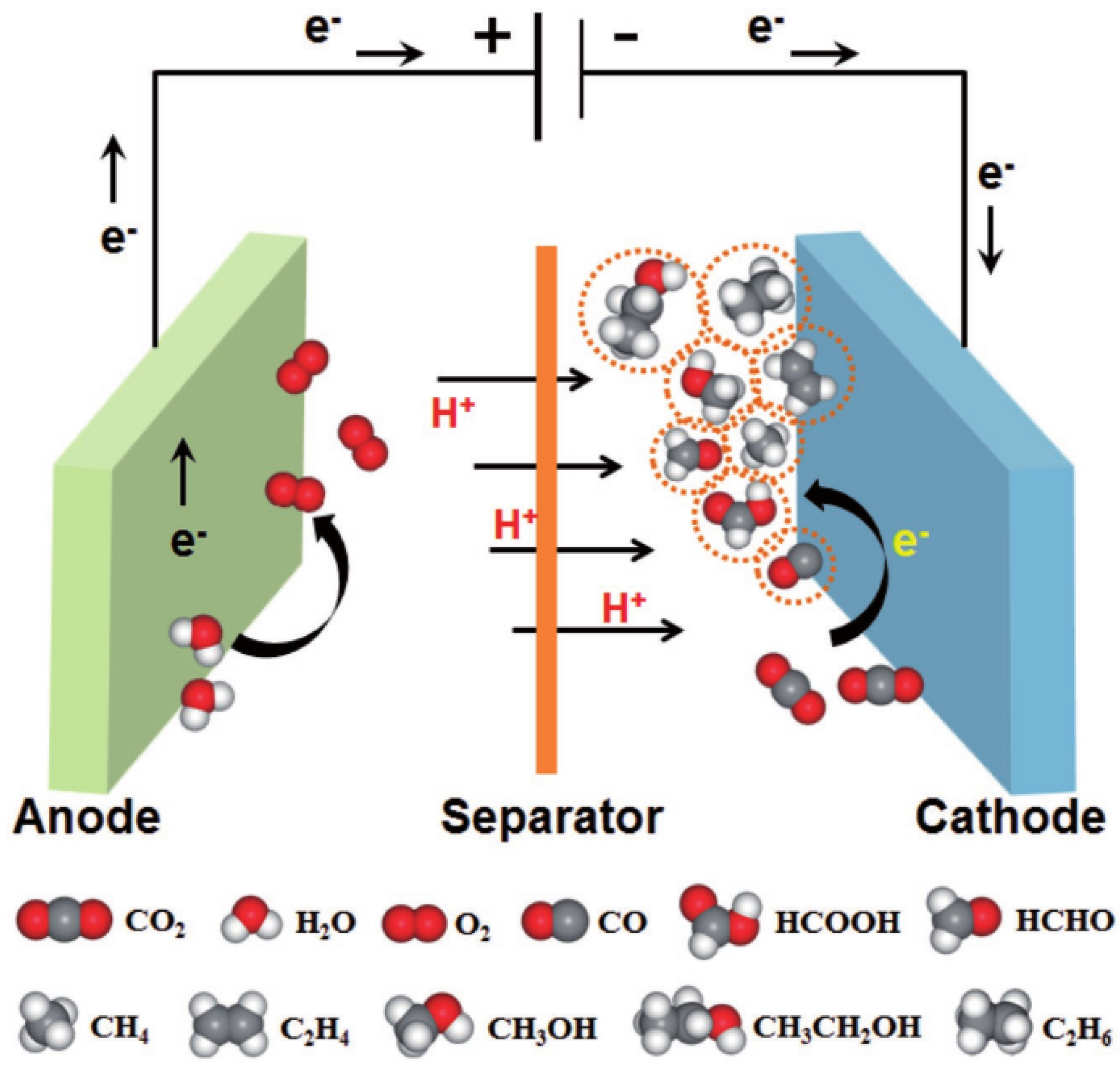
Catalysts Free FullText Electrochemical Reduction of Carbon Dioxide Recent Advances on Au
The formation of manganese oxide is the main side reaction on anode. This side reaction is hard to avoid as it has almost the same electrochemical potential (∼1.23V SHE) as the Oxygen evolution reaction (OER).In industry, every ton of electrolytic manganese produced is inevitably accompanied with 50∼80 kg manganese dioxide as anode slime, which cause troublesome problems in practical.. Recycling lead from wasted lead acid batteries is related to not only the sustainable development of lead-acid battery industry, but also the reduction of the lead pollution to the environment.. The current efficiency is as high as 99.9% and the electrolytic lead recovery rate reaches up to 99.8%. In addition, the new recovery process avoids.