Question: Consider the reaction Ca(OH)2(aq) + 2 HCl(aq) CaCl2(s) + 2 H2O(l) Using the standard thermodynamic data in the tables linked above, calculate the equilibrium constant for this reaction at 298.15K.. Reaction of Ca(OH) 2 and HCl and balanced equation As mentioned earlier, calcium chloride and water are given as products. Because Calcium hydroxide's solubility is low in water, Ca(OH) 2 partially dissociates to Ca 2+ and OH-ions. Therefore there is very low OH-concentration in the aqueous solution which contains Ca(OH) 2.; But, HCl is readily soluble in water dissociates to H + ions and Cl.

SOLVED which is the correct set of products for the acid base neutralization reaction between

resolver con el método de tanteo HCl + Ca(OH)2 → CaCl2 + H2O ayudenme xfi Brainly.lat

How to Balance Ca + H2O = Ca(OH)2 + H2 (Calcium plus Water) YouTube

Ca(OH)2 + HCl > CaCl2 + H2O Brainly.in
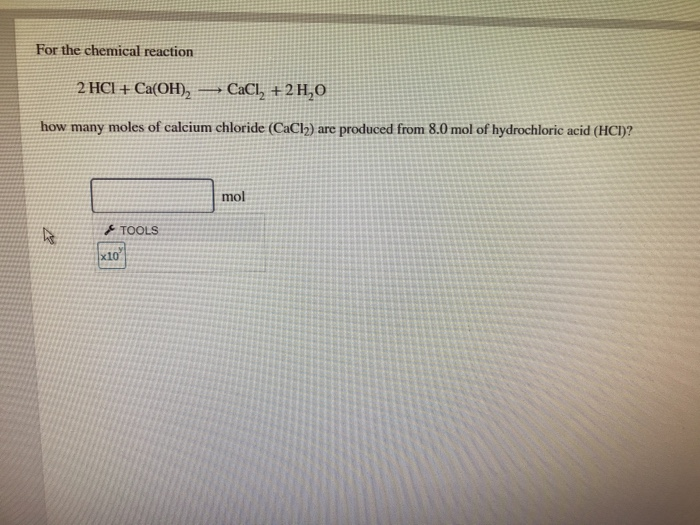
Solved For the chemical reaction 2HCI + Ca(OH)2→CaCl2+2H2O

CaO+HCl=CaCl2+H2O Balanced EquationCalcium oxide+Hydrochloric acid=Calcium chloride+Water
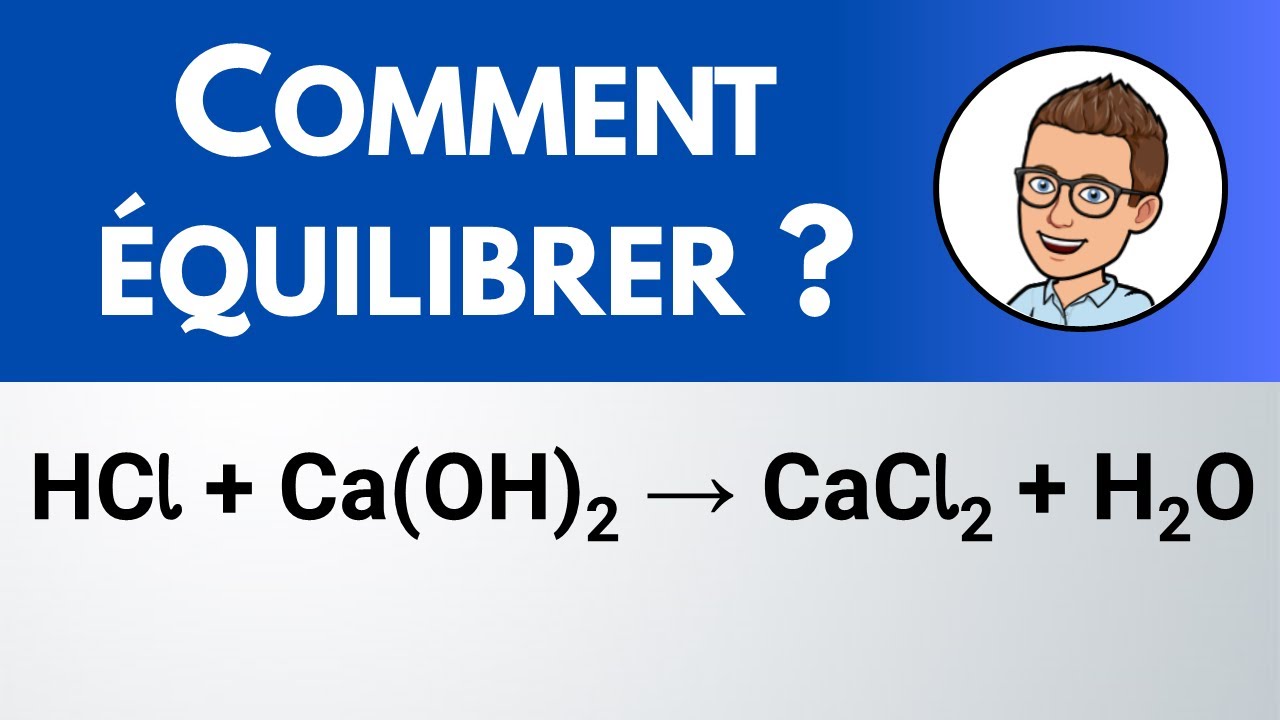
Comment équilibrer ? HCl + Ca(OH)2 + CaCl2 + H2O YouTube
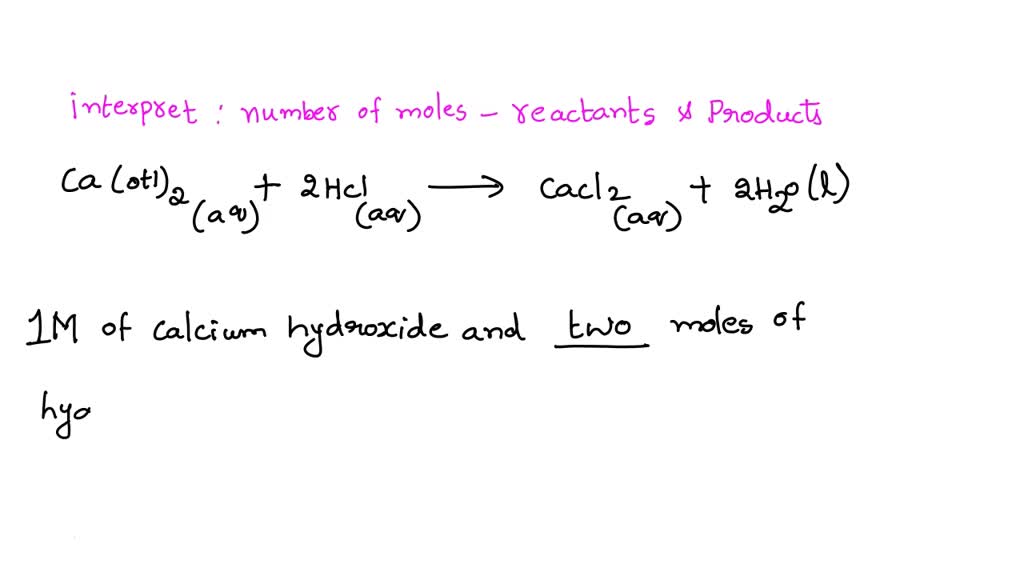
SOLVED The balanced chemical equation for the reaction between calcium hydroxide and

Experimental and modeled total volume change for Ca(OH)2CaCl2H2O... Download Scientific Diagram

Equation for Calcium Hydroxide Dissolving in Water Ca(OH)2 + H2O YouTube

How to balance HCl+Ca(OH)2=CaCl2+H2OChemical equation HCl+Ca(OH)2=CaCl2+H2OHCl+Ca(OH)2=CaCl2
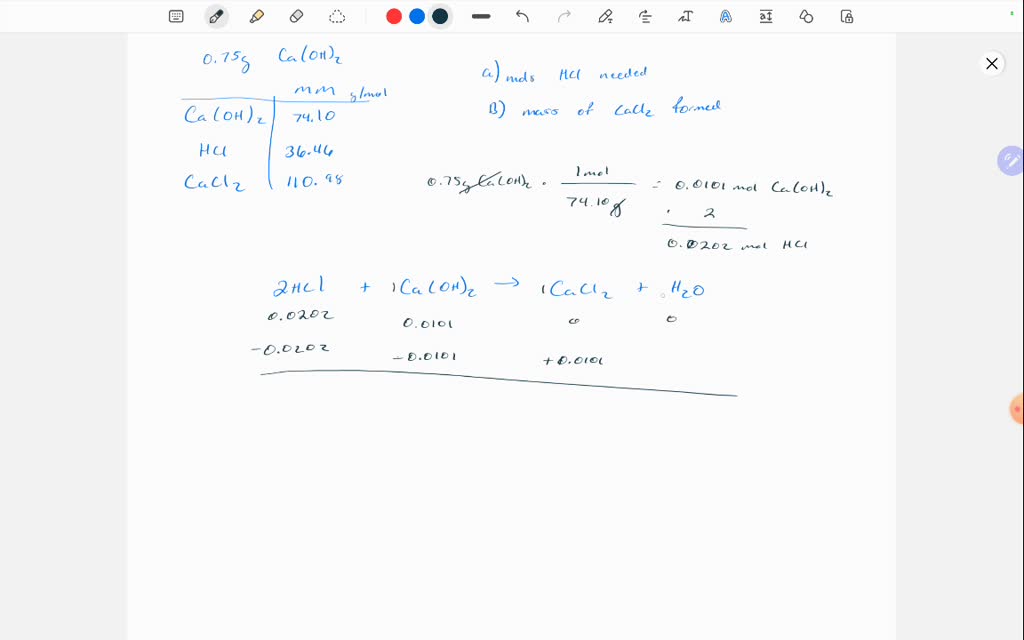
SOLVED In the chemical reaction below, calcium (Ca) and water (H2O) react to form calcium

5 Phase isopleth section of the Ca(OH) 2 CaCl 2 H 2 O system with... Download Scientific Diagram

How to Write the Net Ionic Equation for CaO + HCl = CaCl2 + H2O YouTube

Titration of HCl and Ca(OH)2 YouTube

Synthesis of Ca(OH)2 Calcium Hydroxide YouTube

2 Phase isopleth of (Ca(OH)2 CaCl2 H2O) ternary system (Qiao et al.,... Download Scientific

SOLVED Calcium oxide reacts with water in a combination reaction to produce calcium hydroxide

CaCl2Ca(OH)2H2O phase diagram for different Ca(OH)2/CaCl2 molar... Download Scientific Diagram

How to balance Ca(OH)2 + HCl → CaCl2 + H2O YouTube
Balance the following equation:Ca(OH)2 + HCl → CaCl2 + H2OSUBSCRIBE if you'd like to help us out!https://www.youtube.com/channel/UC2C34WdYMOm47PkWovvzLpw?sub.. In fact, we can even be more concise than this because the calcium salts are present in solution as Ca^ (2+) (aq) and X^ (-) (aq), and these are simply along for the ride. Accordingly, we could write the net ionic equation as, OH^ (-) + H^ (+) rarr H_2O We must still observe stoichiometry, and remember that the molar quantity of calcium.